The European Union has provided additional guidance for veterinary vaccines, narrowing the regulatory gap with human vaccines, notably by identifying an adjuvant by the trade name and not the substance name. Certification of Good Manufacturing Practices is becoming more relevant. Veterinary Clinical trials may be registered in the USA, Canada, and EU.
Q-VANT supports the evolving needs of veterinary vaccine manufacturer.
Focusing on regional or local diseases and emergencies.
Developing affordable and safe adjuvants for farm and companion animal immunizations.
Veterinary quality and regulatory compliance requirements closely adhere to the requirements for human vaccines.
Providing a dossier for upgrading the source of saponin adjuvant in currently marketed vaccines and future developments.
Supporting procedures for quality and regulatory alignment across organizations.

Quality and Regulatory Requirements for Veterinary vaccines.
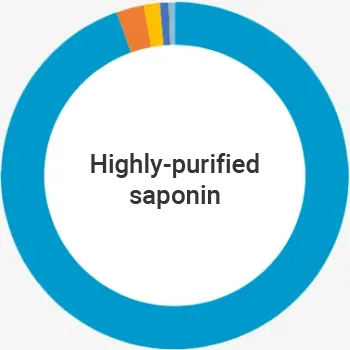
Q-VANT recommends the use of highly purified and affordable saponin adjuvants for veterinary vaccines.
QSap™ Adjuvant
Second generation of Saponin adjuvants for veterinary vaccines.
QSap™ characteristics:
Sample for animal trials
SPI Pharma, our strategic partner can deliver QSap™ adjuvant for animal trials. Samples are readily available.
QSap™ adjuvant offers the guarantee of a secure supply and volume for vaccines.
Q-VANT team can provide expertise on vaccine formulations with QSap™ adjuvant.