QS-21 INFINITY is a next-generation saponin-based adjuvant designed to support innovation in vaccine development. Developed through our proprietary Q-SAP® technology platform, it offers consistent quality, regulatory clarity, and scalable supply, ensuring confidence from preclinical studies to large-scale production.
Key Characteristics
- High Purity: It contains over 95% pure QS-21 molecules, ensuring high performance and consistency in your formulations.
- Form: Delivered as a lyophilized powder for ease of handling and storage.
- Validated Profile: HPLC-verified and validated against tree bark–derived QS-21 to ensure identity and equivalence.
- Regulatory Confidence: Produced from Quillaja biomass using standardized processes. As the same active molecule, no regulatory gap is anticipated.
Available for Research: Samples are readily available for evaluation.
Proliferation of CD4+ T cells re-stimulated with OVA
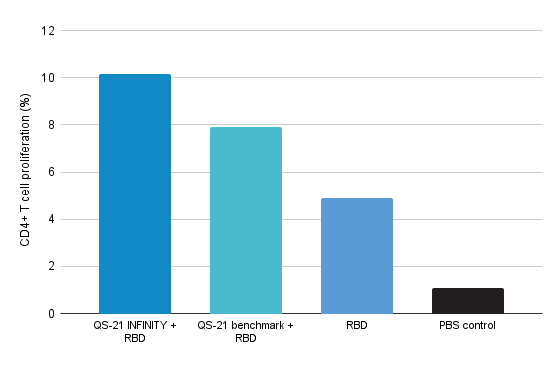
Activated CD4+ T cells expressing CD25
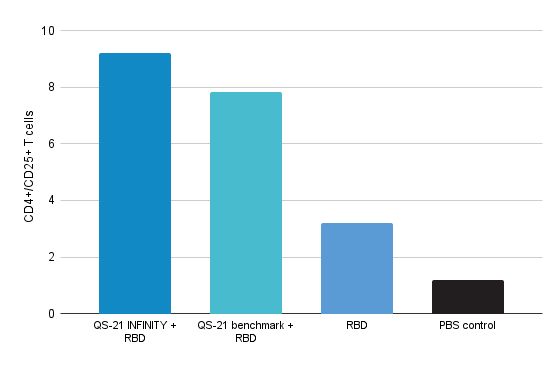
- QS-21 INFINITY standalone potentiates the proliferative capacity and activation of CD4+ T cells and antigen specific CD4 + T cells.
- QS-21 INFINITY standalone shows statistically none or slightly better performance than benchmark.
Antibody levels induced by immunization with OVA and our saponin-based adjuvant.

- QS-21 INFINITY standalone induces higher antibody level than the antigen alone.
- QS-21 INFINITY standalone matches the performance of benchmark.

Partnership with SPI Pharma
Our strategic partner, SPI Pharma, facilitates access to samples for preclinical studies. With a strong focus on pharmaceutical innovation, SPI Pharma supports your R&D needs with fast and reliable sample delivery.

Formulation Support
The Q-VANT Biosciences team brings extensive expertise in vaccine formulation using saponin-based adjuvants. We offer guidance and technical insight to help you unlock the full potential of your research and development programs.

Why QS-21 INFINITY?
- Secure Supply Chain: A reliable, scalable, and sustainable source of high-purity saponin-based adjuvant.
- Regulatory Readiness: Structurally identical to traditional Quillaja-derived compounds, helping streamline approval processes.
- Formulation-Ready: Lyophilized and quality-assured—ready to integrate into your vaccine development pipeline.
Request a Sample
Explore the future of vaccine adjuvants. Contact us to request your sample and connect with our scientific team.